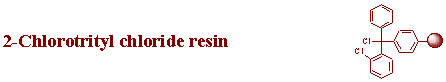
Resins for the Syntheses of Protected Peptide Fragments
by Fmoc Chemistry
There are a few resins for the preparation of protected peptide acid and amide fragments by Fmoc chemistry. However, we recommend our customers to use 2-chlorotrityl chloride resin for synthesis of protected peptide acid fragments, and Sieber Amide resin for protected amide fragments. Those two resins provide the peptides in high yield and purity.
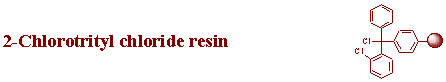
|
11001 |
2-Chlorotrityl chloride resin Substitution: 0.8 - 1.6 mmole/g resin Bead size 100- 200 mesh (polystyrene- 1% DVB) |
1 g 5 g 25 g 100 g |
$35 $140 $575 $2,300 |
|
11002 |
2-Chlorotrityl chloride resin Substitution: 0.8 - 1.6 mmole/g resin Bead size 200- 400 mesh (polystyrene- 1% DVB) |
1 g 5 g 25 g 100 g |
$35 $140 $575 $2,300 |
Attachment of the Fmoc-amino acid on 2-chlorotrityl chloride resin:
In a typical experiment, Fmoc-amino acid (6 mmole) and DIEA (4.4 ml, 25 mmole) are dissolved in dry dichloromethane (100 ml). A small amount of DMF is added if the acid is not completely dissolved in DCM (just enough to facilitate dissolution of the acid). The resulting solution is added to 2-chlorotrityl chloride resin (10 g, 1.1 - 1.6 mmole/g). The suspension is stirred for other 20 minutes. Subsequently methanol (10 ml) is added, and stirring is continued for other 10 minutes. The resin is filtered, washed (with 3 x DCM, 2 x DMF, 2 x methanol), and dried in vacuum. The final substitution rate can be determined photometrically from the amount of Fmoc chromophore released upon treatment of the resin with piperidine/DMF. For long term storage of the pre-loaded resin, the Fmoc group should be removed as it has been found to be more stable in the basic form.Cleavage of protected peptide from the resin:
The peptide synthesis on 2-chlorotrityl resin can be achieved by the regular Fmoc protocol with either DIC/HOBt or HBTU/DIEA activation and coupling. The resulting protected peptide fragment can be obtained by treating the resin with HOAc/TFE/DCM [1], 0.5% TFA or HFIP [2]. The cleavage conditions tolerate the side chain protecting groups used for regular Fmoc chemistry.[1] K. Barlos, et al., (a)Tetrahedron Lett., 1989, 30, 3943 & 3947;
(b) Int. J. Peptide Protein Res., 1991, 37, 513; 38, 555 & 562;
(c) Angew. Chem., 1991,103,572.
[2] R. Bollhage, et al., J. Chem. Soc., Chem. Commun., 1994, 2559.
![]()
|
14001 |
9-Fmoc-amino-xanthen-3-yloxy-Merrifield resin Substitution: 0.25 - 0.6 mmole/g resin Bead size 100- 200 mesh (polystyrene- 1% DVB) |
1 g 5 g 25 g |
$70 $285 $980 |
Cleavage of protected peptide from the resin:
The peptide fragment can be obtained by treating the resin with 1% TFA (P. Sieber, Tetrahedron Lett., 1987, 28, 2107).
|
|
|
|
|